Midwifery education

 After 11 years of Workshops and Intensives at Birthspirit Cottage in Tamahere, and around New Zealand and Australia, Birthspirit has taken its education online with easy to use technology. Midwives have loved the extensive resources, setting their own timetables, and connecting with Maggie and others around the world in the live classrooms and discussion forums. As one midwife put it: “It allows midwives to engage in high quality education that is achievable whether you are urban/rural; NZ or UK!!”
After 11 years of Workshops and Intensives at Birthspirit Cottage in Tamahere, and around New Zealand and Australia, Birthspirit has taken its education online with easy to use technology. Midwives have loved the extensive resources, setting their own timetables, and connecting with Maggie and others around the world in the live classrooms and discussion forums. As one midwife put it: “It allows midwives to engage in high quality education that is achievable whether you are urban/rural; NZ or UK!!”
WORKSHOPS
Vaginal Birth after Caesarean Section (VBAC) Workshop (Online)
 The maternity experience in New Zealand results in a 26.2% caesarean section rate for women and their babies, with over half performed as labour emergencies…read more
The maternity experience in New Zealand results in a 26.2% caesarean section rate for women and their babies, with over half performed as labour emergencies…read more
ARTICLES & POSTS
The obstetric bed: resistance in action
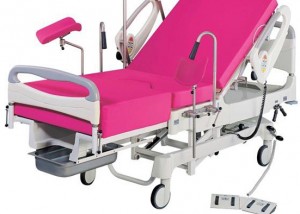 Bedbirthing has predominated in Western countries reputedly since it gained acceptance after Louis XIV’s mistresses birthed in bed; the king covertly watched from behind…read more
Bedbirthing has predominated in Western countries reputedly since it gained acceptance after Louis XIV’s mistresses birthed in bed; the king covertly watched from behind…read more
Breech Birth Online Workshop
 Breech presentation is the 4th most commonly reported indication for caesarean section, and previous caesarean section the first. Breech presentation can be seen…read more
Breech presentation is the 4th most commonly reported indication for caesarean section, and previous caesarean section the first. Breech presentation can be seen…read more
Insights through the murk of meconium
 As I compiled the statistics of my home birth practice covering a 22 year period many of the individual instances of how my knowing developed became definable…read more
As I compiled the statistics of my home birth practice covering a 22 year period many of the individual instances of how my knowing developed became definable…read more
Post-dates Pregnancy Workshop (Online)
 In 1985, in order to stem the tide of increasing medical intervention during childbirth, the WHO determined that no geographical region should have an induction of labour rate…read more
In 1985, in order to stem the tide of increasing medical intervention during childbirth, the WHO determined that no geographical region should have an induction of labour rate…read more
Keep on singing when life’s a breech
 As steeped in medically and midwifery managed birthing as this 60-plus year old breech story is, it has (at least) three central themes – a woman who was confident about giving birth…read more
As steeped in medically and midwifery managed birthing as this 60-plus year old breech story is, it has (at least) three central themes – a woman who was confident about giving birth…read more
The Spirit of Birth: Nature, Nurture and the Evidence (Online)
 In the last three decades, women in New Zealand have experienced escalating levels of intervention during childbirth and a declining ‘normal’ birth rate…read more
In the last three decades, women in New Zealand have experienced escalating levels of intervention during childbirth and a declining ‘normal’ birth rate…read more
Water birth in New Zealand : herstory and politics
 Using deep warm baths in labour is a common strategy that many home birth midwives have used for at least three decades in New Zealand to promote relaxation and comfort …read more
Using deep warm baths in labour is a common strategy that many home birth midwives have used for at least three decades in New Zealand to promote relaxation and comfort …read more
Water Birth Workshop (Online)
 Since the first of the modern water births in New Zealand over three decades ago, water birth has been embraced by women as a popular choice to facilitate drug-free labours…read more
Since the first of the modern water births in New Zealand over three decades ago, water birth has been embraced by women as a popular choice to facilitate drug-free labours…read more
The FTPs of caesarean section
 When Nadine gave birth at home to her second baby, a daughter, she fulfilled a dream of natural childbirth and immediate, uninterrupted in-arms mothering. …read more
When Nadine gave birth at home to her second baby, a daughter, she fulfilled a dream of natural childbirth and immediate, uninterrupted in-arms mothering. …read more
WISE WOMAN ARCHIVES TRUST (INC)
 Birthspirit continues to fully support Wise Woman Archives Trust Inc but it now has its own website. To find out more about the Trust and maternity history in New Zealand see here.
Birthspirit continues to fully support Wise Woman Archives Trust Inc but it now has its own website. To find out more about the Trust and maternity history in New Zealand see here.
Mamatoto calling
 Each of Jaynie’s three labours built up over several days before she had contractions that were frequent enough for her to determine she was in labour…read more
Each of Jaynie’s three labours built up over several days before she had contractions that were frequent enough for her to determine she was in labour…read more
A toe in the water: exploring breech waterbirth
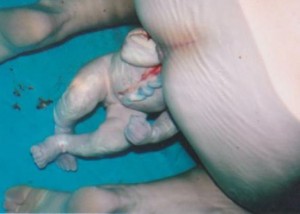 I am often asked during breech workshops that I run if leaving a woman in a birth pool to give birth to a surprise breech baby, undiagnosed until on the perineum, was ‘reasonable’…read more
I am often asked during breech workshops that I run if leaving a woman in a birth pool to give birth to a surprise breech baby, undiagnosed until on the perineum, was ‘reasonable’…read more
